Beurer EM38: ENGLISH
ENGLISH: Beurer EM38

ENGLISH
Contents
1. About the unit ........................................................11
6. Operation ...............................................................16
2. Signs and symbols ................................................11
7. Cleaning and care of the unit and belt ..................17
3. Important notes .....................................................12
8. Disposal .................................................................17
4. Unit description .....................................................14
9. Troubleshooting .....................................................17
5. Using the unit for the first time and changing the
10. Technical specifications ......................................18
batteries .................................................................15
Included in delivery
•
Back belt with control unit EM 38
•
Extension belt
•
Batteries, 3 x 1.5 V AAA (LR03, micro)
•
This instruction manual
1. About the unit
Dear valued customer
Thank you for choosing one of our products.
Our name stands for high-quality, thoroughly tested products for applications in the areas of heat, weight, blood
pressure, body temperature, pulse, gentle therapy, massage and air.
Please read these instructions for use carefully and keep them for later use, be sure to make them accessible to
other users and observe the information they contain.
With kind regards
Your Beurer team
Pain relief on lower back with the EM 38 stimulation current unit
How does stimulation current work?
The back belt works on the basis of electrical nerve stimulation (TENS).
The more precise term is transcutaneous (meaning “through the skin“) electrical nerve stimulation (TENS). TENS
is approved as a clinically proven, eective, medication-free method of treating certain types of pain which,
when used properly, is also free of side eects. It is also approved as a means of simple self-treatment.
The pain-relieving or pain-suppressing eect is caused by suppressing the transmission of pain in the nerve
fibres (particularly through high-frequency impulses) and increasing the release of the body‘s own endorphins,
which decrease the sensation of pain by their eect in the central nervous system. This method is clinically
tested and approved.
Every condition that calls for the use of TENS must be clarified by your attending physician. He or she will also
give you information about the specific benefits of a TENS self-treatment.
2. Signs and symbols
The following symbols appear in these instructions.
Warning Warning instruction indicating a risk of injury or damage to health.
Important Safety note indicating possible damage to the unit/accessories.
Note Important information to be noted.
11
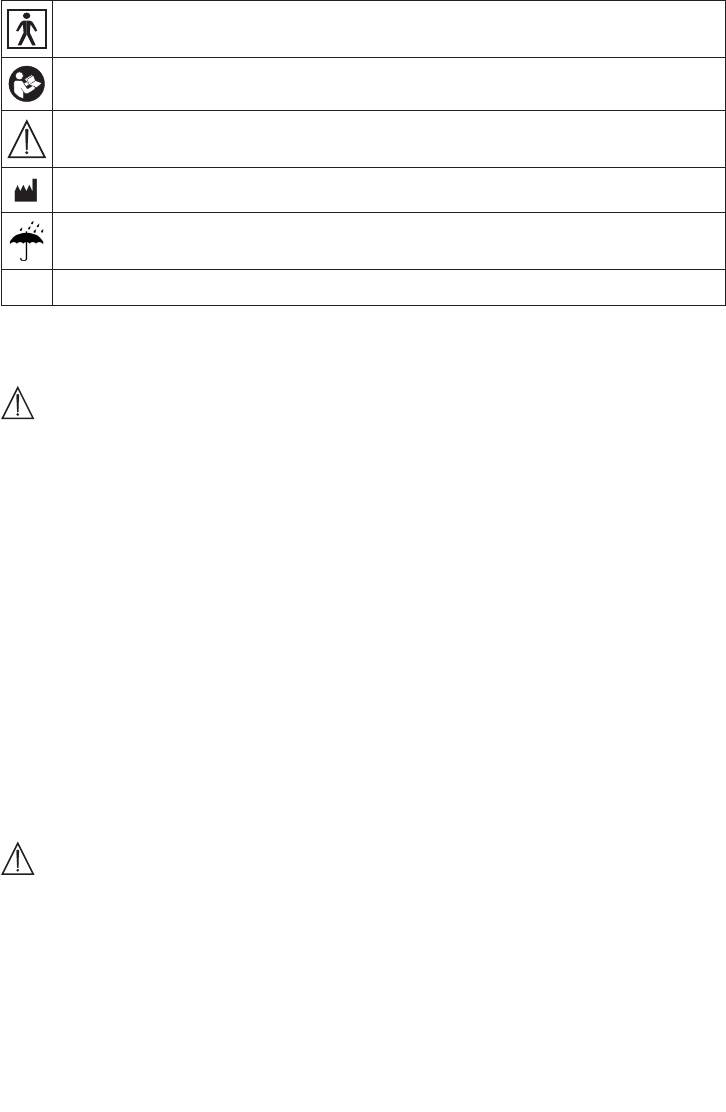
The following symbols are used on the identification plate.
Type BF applied part
Refer to instructions for use
Devices that can emit eective output values above 10mA or 10V, averaged over every 5 second
interval.
Manufacturer
Keep dry
12
SN
Serial number
3. Important notes
Safety notes
Warning
•
Use the belt only:
– on humans,
– for external use,
– for the purpose for which it was developed and in the manner specified in these instructions for use.
•
Any improper use can be dangerous!
•
In acute emergencies, first aid takes priority.
•
This unit is not intended for commercial or clinical use, but only for individual, private household use!
•
Before use, ensure that there is no visible damage to the unit or accessories. When in doubt, do not use the
unit and contact your dealer or the customer service address provided.
•
If you have health concerns of any kind, contact your general practitioner!
•
Use the unit in the lower back area only! Using it on other body parts can cause severe health problems.
•
A slight reddening of the skin after use is normal and disappears after a short time. Refrain from using the unit
until the reddening has gone away.
•
If skin irritation results from a long therapy time, select a shorter application time.
•
In case of more severe skin irritation, discontinue treatment and consult a physician.
•
This unit is not intended for use by children or persons with physical, sensory (e.g. insensitivity to pain) or
mental impairments or those who do not know how to use it due to a lack of experience or knowledge, unless
they are supervised by a person responsible for their safety or have received instructions from them on how to
use the unit.
•
Keep children away from packaging materials (risk of suocation).
•
Do not use attachments that are not recommended by the manufacturer.
Safety precautions
Warning
•
Carry out the first few minutes of treatment while sitting or lying down, so that if you experience a rare vasova-
gal reaction (i.e. you feel faint), you are not at unnecessary risk of injury. If you start to feel faint, shut o the
unit immediately and elevate your legs (for approx. 5 – 10 min.).
•
The treatment should be pleasant. If the unit does not function correctly or if you begin to feel unwell or feel
pain, stop using the unit immediately.
•
Take o the belt only when the unit is switched o!

Notes about the electrodes
Important
•
Never apply the electrodes to injured skin areas.
2
•
Max. recommended initial value for electrodes: 5 mA/cm
.
2
•
Eective current density levels over 2 mA/cm
require increased attention on the part of the user.
Warning
To prevent harm to your health, we urgently recommend that you do not use the unit in the following
situations:
•
Do not use the unit if you use a pacemaker or other implants, such as an insulin pump or metallic
implants.
•
If you have a high fever (e.g. > 39 °C).
•
If you have known or acute arrythmias or other problems involving cardiac stimuli and their transmission to the
heart.
•
If you have seizure disorders (such as epilepsy).
•
If you are pregnant.
•
If you have cancer.
•
After operations in which increased muscle contractions could interfere with the healing process.
•
On acutely or chronically diseased (injured or inflamed) skin, e.g. painless or painful inflammation or reddening.
•
If you have rashes (e.g. allergies), burns, bruises, swelling and open wounds or those in the process of healing.
•
On surgical scars in the process of healing.
•
When simultaneously connected to a high-frequency surgical instrument. In this case, burns under the
stimulation current fields can result.
•
When under the influence of pain medication, alcohol or sleep aids.
•
Whenever doing anything in which an unforeseen reaction (such as increased muscle contraction despite low
intensity) can be hazardous, for example while driving a car or operating machinery.
•
On sleeping persons.
•
Do not use this unit simultaneously with other units that transmit electrical pulses to your body.
•
The unit is suitable for private, individual use.
•
For hygienic reasons, the belt may be used by one person only.
•
Ensure that during stimulation, no metallic objects can contact the electrodes, as otherwise localised burns
can occur.
The unit must not be used in the following situations:
•
In the head area: Here, it can trigger cramping.
•
In the neck/carotid artery area: Here, it can trigger cardiac arrest.
•
In the throat and larynx area: Here, it can trigger muscle cramps that can cause suocation.
•
In the ribcage area: Here, it can increase the risk of ventricular fibrillation and cause cardiac arrest.
Safety precautions
•
Do not use the unit in close proximity to shortwave or microwave units (e.g. cell phones), as these can cause
fluctuations of the initial values of the unit.
•
Do not use while sleeping, driving a motor vehicle or simultaneously operating machinery.
•
Never immerse the unit in water or other liquids.
•
Do not use in close proximity to highly inflammable materials, gases or explosives.
Consult a doctor before use in the following situations:
•
Acute diseases, particularly in case of suspected or known blood coagulation disorders, susceptibility to
thromboembolitic disease or malignant growths.
•
If you have diabetes or other diseases.
•
Chronic pain conditions that have not been clarified, regardless of the region of the body.
•
Any sensitivity disorders with reduced sensation of pain (such as metabolic disorders).
•
Simultaneously carried out medical treatments.
•
Discomfort experienced as a result of the stimulation treatment.
•
Persistent skin irritation under the electrodes.
Warning
Using the unit is not a substitute for consultation or treatment from a physician.
Whenever you have any pain or illness, always contact your physician first.
13
Before using the unit for the first time
Important
•
Before you use the unit for the first time, remove all packaging materials.
•
Switch the unit o immediately if it is defective or malfunctioning.
•
If the self-adhesive covers for the electrode connection are missing or become detached, we recommend
urgently that you ax the electrode covers provided.
•
Never wear the belt with bare metal electrodes. Using the belt without electrode covers can cause injuries.
Important
•
The manufacturer shall not be held liable for damage or injuries caused by improper or incorrect use.
•
Protect the unit from dust, dirt and moisture.
•
If the unit has fallen or been dropped, exposed to extreme moisture or incurred other types of damage, it must
no longer be used. Do not expose the unit to high temperatures or direct sunlight.
•
Never open or attempt to repair the unit by yourself, as otherwise proper function is no longer guaranteed.
Failure to observe this regulation shall void the warranty.
•
If you need to have the unit repaired, contact customer service or an authorised dealer.
Notes on handling batteries
•
Keep batteries out of the reach of children. Children can put batteries in their mouth and swallow them. This
can cause severe harm to their health. In this case, consult a physician immediately!
•
Normal batteries must not be recharged, heated or thrown into an open flame (danger of explosion!).
•
Always replace all batteries at the same time and use batteries of the same type.
•
Leaking batteries can damage the unit. If you do not intend to use the unit for a prolonged period, remove the
batteries from the battery compartment.
•
Leaky or damaged batteries can cause caustic injuries if they touch the skin. In this case, wear suitable safety
gloves.
4. Unit description
Overview of the control unit
14
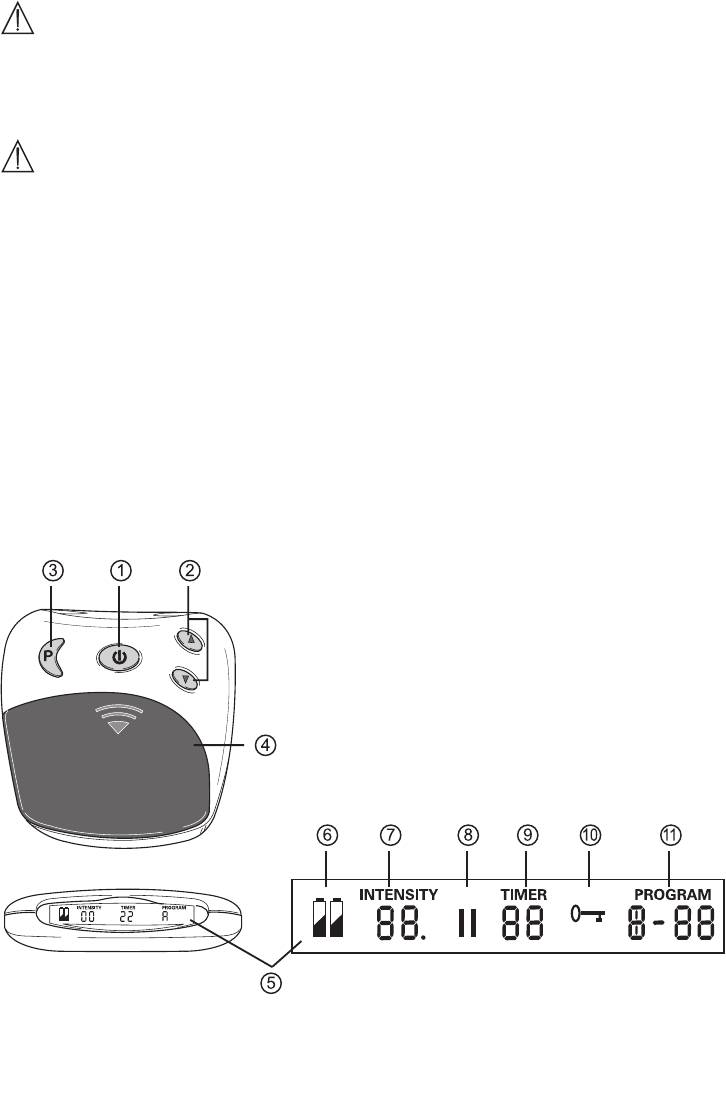
Item Item Designation Item Item Designation
1 On/o/pause button 6 Battery status, appears when batteries are used up
2 Intensity adjustment
7 Intensity, level 0 – 20
increase
8 Pause symbol, flashes when activated
decrease
3 Program/locking key 9 TIMER: Remaining time of the active programs in
minutes
4 Battery compartment 10 Keylock active
5 Display with LCD display 11 Active program
5. Using the unit for the first time and changing the batteries
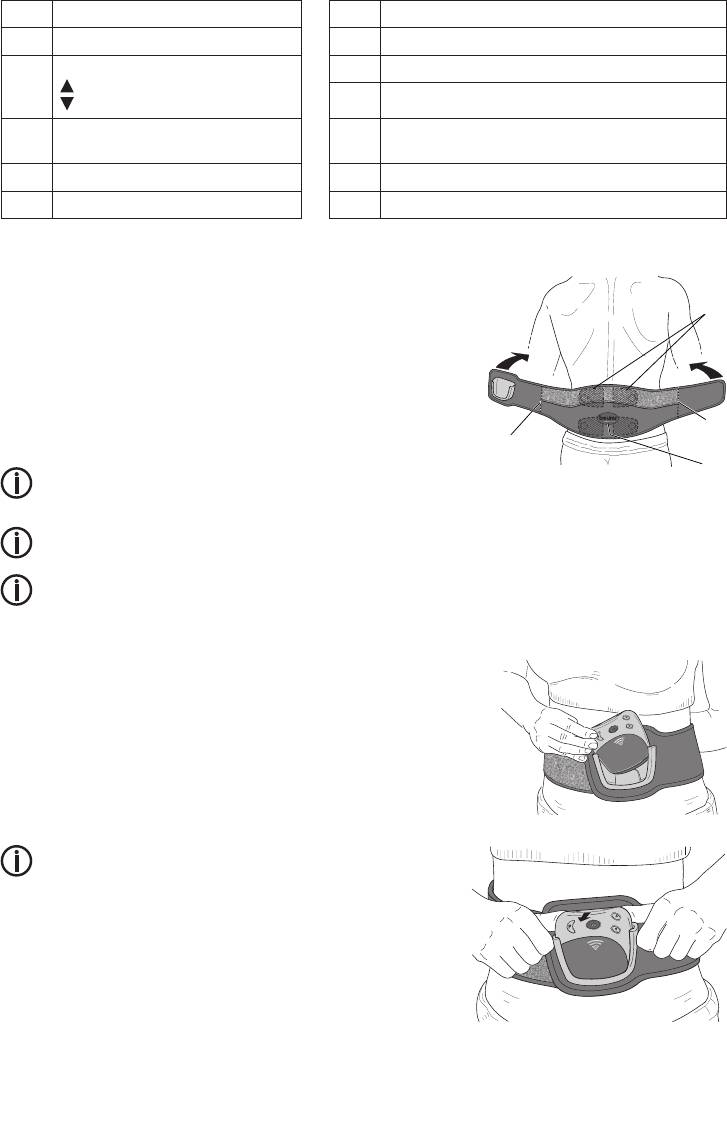
Putting on the belt
Before each use, ensure that your skin is clean and free of creams or oils.
Only in this way the unit can work optimally.
•
Lay out the belt before you with the electrodes facing you.
•
Moisten the electrodes and your back with water. Ensure that the elec-
trode surfaces are moistened evenly to avoid localised voltage peaks
when they are applied to your skin.
•
Fit the belt around your waist so that the vertical seams A are at the
same place on each side of your body. The Beurer logo B is then in the
middle of the spinal column.
See the diagram.
When positioned correctly, the electrodes C are slightly to the left and right of your spinal column.
• Then, close the Velcro fastener.
Ensure that the belt fits tightly enough to provide a good contact between the electrodes and your skin, but
does not constrict.
Extending the belt
If the belt is not adequate for your waistline, you can use the extension belt provided. Depending on your
waistline, the holder for the control unit may be away from the centre of your body. It is important that the
belt is positioned correctly, therefore the electrodes C must be to the left and right of the spinal column.
Inserting the control unit
•
Insert the control unit into the holder on the belt.
•
The operating keys should be facing outwards and the display should be
facing upwards and towards you.
•
If inserted correctly, the control unit clicks into the holder.
Automatic shuto
If the unit is not used after being switched on, it switches o automatically
after 3 minutes.
Changing the batteries
If the battery status indicator [6] flashes, it is time to change the
batteries. To insert or replace batteries,
you have to remove the control unit from the holder of the belt.
•
Release the magnetic connection by gently pulling the control unit
away from the belt. You can feel the magnetic connection of the two
contact buttons unsnap.
•
Pull the unit upwards and out of the holder.
•
Open the battery compartment [4] by pushing the cover downwards
at the arrow mark.
15
B
C
A
A

•
Insert 3 x 1.5 V batteries, type AAA (LR03 Micro). Ensure the correct polarity.
See the diagram inside the battery compartment.
•
Do not use any rechargeable batteries!
6. Operation
General information about use
The back belt is intended for individual treatment of pain in the lower back area.
Do not switch on the unit until the belt is fitted correctly. See item 5.
Switching on the unit
Press and hold down the on/o button [1] until a brief signal sounds and the LCD display [5] switches on.
When the unit is started for the first time, Program A is activated automatically.
Selecting the program
Press the program button P [3] to switch through the programs. The following programs are available:
Program Frequency Time
A 4 Hz –110 Hz (3 phases) 30 min.
B 4 Hz 25 min.
C 2 Hz (burst) 25 min.
D 100 Hz 25 min.
Note:
For program A, you can feel the eect become stronger when switching from phase 1 to phase 2 (after ap-
prox. 10 minutes). This is normal and intentional. If the intensity seems too high, decrease it using the
▼ intensity button [2].
Note:
If you change the program during the stimulation (for example from A to B), the initial intensity in the new
program increases incrementally up to the previously set intensity. This can be stopped if necessary by
pressing the ▼ intensity button [2] for two seconds or by switching o the unit by pressing the on/o but-
ton [1] for two seconds.
Stopping impulses that are too strong
You can decrease the intensity at any time or switch o the unit by pressing the on/o button [1] (approx.
2 seconds).
Adjusting the intensity
Press the ▲ intensity button [2] to increase the intensity incrementally or press the ▼ intensity button [2] to lower
the intensity. You can adjust the intensity in 20 Increments. Depending on the intensity level, you feel a prickling
at first that can escalate to a muscle contraction.
Select a setting that is within your comfort level.
Preventing unwanted pulse changes
To prevent an accidental increase in intensity during a treatment session, simply activate the keylock. To do
so, press the program selection button P [3] for approx. 2 seconds. An acoustic signal sounds and the “
”
symbol appears in the display[5].
To cancel the keylock, press and hold down the P [3] button for approx. 2 seconds.
Reacting to unpleasant sensations
If you feel a stinging or itching sensation, switch o the unit and proceed as follows:
•
Check whether the electrodes are working properly or are defective.
•
Check whether the round cover is still present over the electrode connections.
•
Take o the belt and moisten it completely once again.
•
When refitting the belt, ensure good skin contact and good moistening.
16
Inserting a pause
If you want to take a break or pause a program, press the pause button [1] briefly. An acoustic signal sounds and
the “II” pause symbol flashes in the display [5]. You can resume the program by pressing the button [1] again.
Contact detection
If the electrodes have no contact with the body, the intensity is set automatically to zero. This prevents unwanted
stimulation. The intensity cannot be increased without contact with the body.
Memory function
The unit stores the most recently configured program.
After a battery change, the unit starts with the first program again.
7. Cleaning and care of the unit and belt
Cleaning the unit
•
Remove the batteries from the unit before each cleaning.
•
Clean the unit with a soft cloth moistened slightly with water after use. If the dirt is heavier, you can also mois-
ten the cloth with a mild soap solution.
•
Ensure that no water penetrates the unit. If this should occur, do not use the unit until it is completely dry.
•
Do not use any chemical cleaners or abrasives for cleaning.
Important
The belt and control unit are not suitable for machine washing.
Cleaning the belt
Once you have removed the control unit from the belt, you can clean the belt using a soft cloth. To do so, use a
mild soap solution or liquid detergent. Do not use bleaching agents.
8. Disposal
In the interest of protecting the environment, the unit must not be thrown out with the household waste at the
end of its service life. Dispose of the unit at a suitable local collection or recycling point.
Observe the local regulations for material disposal.
Please dispose of the device in accordance with EC Directive – WEEE (Waste Electrical and Electronic
Equipment). If you have any queries, please contact the appropriate local authorities.
Standard and rechargeable batteries should be disposed of separately from the household waste. As a
consumer, you are legally obliged to return used batteries for proper disposal. You can hand in your used
batteries at public collection points in your district or sales outlets where batteries of this type are sold.
The codes below are printed on batteries containing harmful substances:
Pb = battery contains lead
Cd = battery contains cadmium
Hg = battery contains mercury
The batteries for this unit are free of hazardous substances.
9. Troubleshooting
Problem/question Possible cause/remedy
The unit does not switch on when
1. Ensure that the batteries are inserted correctly and have contact. The
you press the ON/OFF button.
on/o button must be pressed and held down to switch the unit on.
2. If necessary, replace the batteries.
3. Please contact customer service.
No stimulation can be felt. 1. Increase the pulse intensity incrementally.
2. Press the on/o button to restart the program.
3. The batteries are almost empty. Replace them.
17
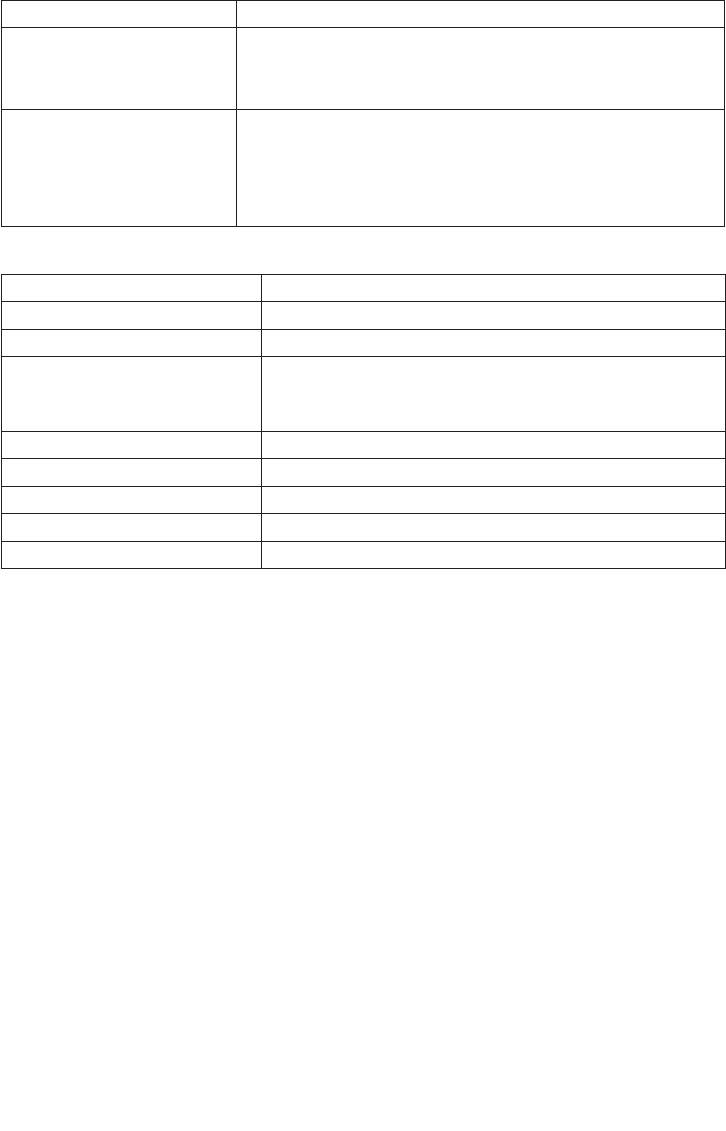
Problem/question Possible cause/remedy
You feel an unpleasant sensation
The electrodes are poorly moistened. This can lead to skin irritation, as
from the electrodes.
the current is no longer distributed evenly over the entire surface. Switch
o the unit. Take o the belt, moisten the belt (electrodes) and your lower
back and restart the program according to the manual.
The skin in the treatment area
Discontinue the treatment immediately and wait until the skin condi
-
becomes red.
tion has normalised. Temporary skin reddening under the electrode is
harmless and can be explained by the localised increase in blood flow.
However, if the skin irritation persists and you feel itching or irritation,
consult your doctor before continuing use. The cause may be an allergy
to the surface of the electrode.
10. Technical specifications
Belt size for waist sizes from Approx. 75 to 140 cm
Weight (belt and unit) Approx. 415 g with batteries
Electrode size Approx. 81 x 65 mm
Parameters (500 Ohm load) Output voltage: max. 50 Vpp / 5.5 Vrms
Output current: max. 100 mApp / 11 mArms
Output frequency: 2 – 110 Hz
Pulse duration 60 – 220 μs per phase
Wave form Symmetric, biphasic rectangular pulses
Power supply 4.5 V (3 x 1.5 V AAA, type LR 03)
Operating conditions 0 °C to 40 °C, 20 to 65% relative humidity
Storage 0 °C to 55 °C, 10 to 90% relative humidity
We reserve the right to make any technical alterations that are necessary in order to improve and develop the
product further. If the unit is used outside of the specifications, proper function is no longer guaranteed!
This unit complies with European standards EN60601-1, EN60601-1-2 and is subject to special precautions
regarding electromagnetic compatibility. Note that portable and mobile RF communication equipment can aect
this unit. For detailed information, contact customer service at the address provided.
The unit conforms to the requirements of the European Directive for Medical Products 93/42/EC, the MPG (Ger
-
man Medical Devices Act). In accordance with the MPBetreibV (German Medical Device Operator Ordinance),
regular metrology checks must be performed if the unit is used for commercial or business purposes. Even for
private use, we recommend having the manufacturer perform a metrology check every 2 years.
18




