ResMed VPAP IV ST: инструкция
Раздел: Товары для здоровья
Тип:
Инструкция к ResMed VPAP IV ST
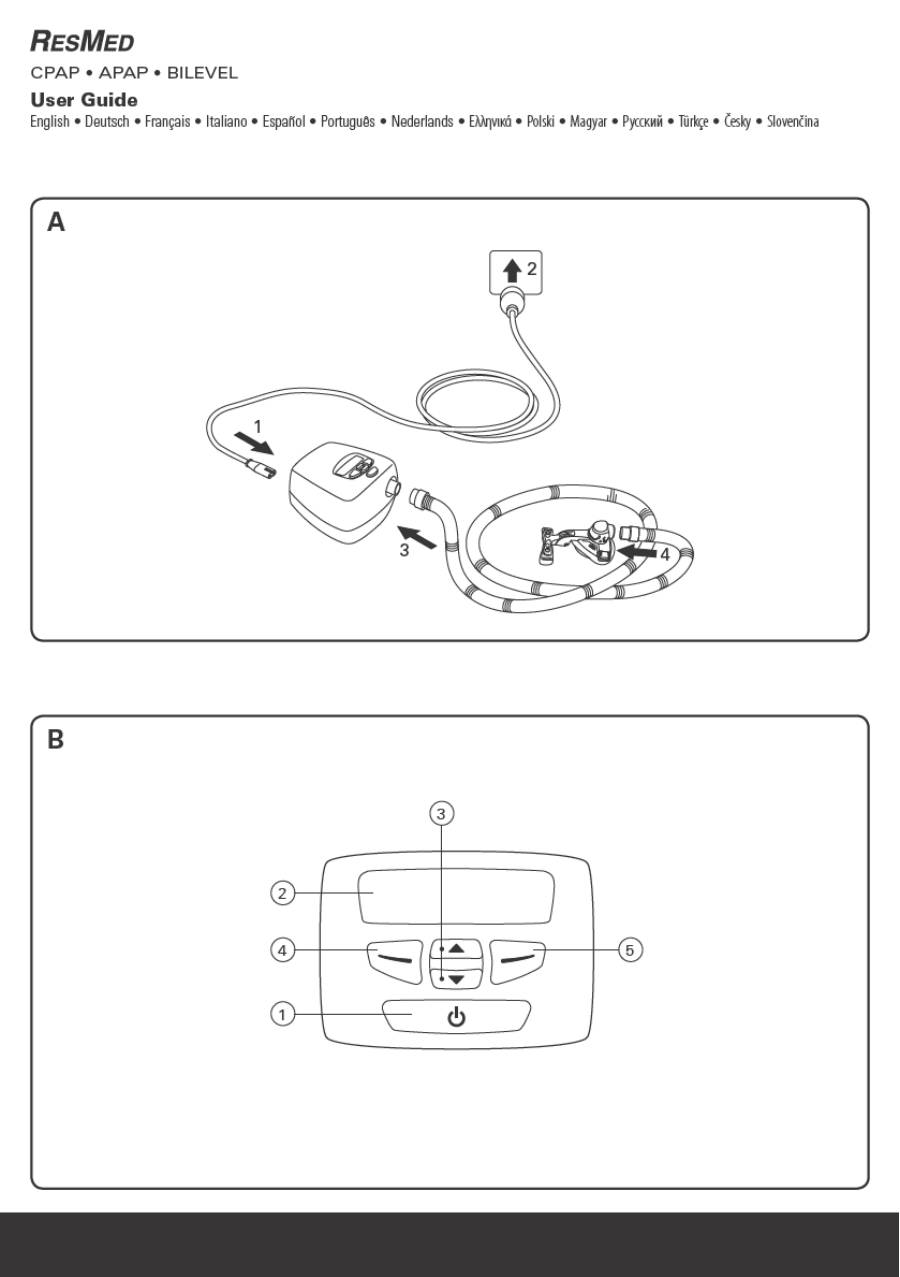


4
Explanation:
When the flow generator is not in operation and
the oxygen flow is left on, oxygen delivered into the air delivery
tubing may accumulate within the flow generator enclosure
and create a risk of fire. This applies to most types of positive
airway pressure devices.
• Oxygen supports combustion. Oxygen should not be used
while you are smoking or in the presence of an open flame.
• Always ensure airflow is being generated by the device before
the oxygen supply is turned on.
• Always turn the oxygen supply off before stopping the airflow
from the device.
Note:
At a fixed rate of supplemental oxygen flow, the inhaled
oxygen concentration will vary, depending on where the
oxygen is introduced, the pressure settings, patient breathing
pattern, mask selection, and leak rate.
• Do not use the flow generator if there are obvious external
defects, unexplained changes in performance or unusual
noises.
• Do not open the flow generator case. There are no user
serviceable parts inside. Repairs and internal servicing should
only be performed by an authorised service agent.
• Explosion hazard—do not use in the vicinity of flammable
anaesthetics.
• The flow generator should not be used with anaesthetised
patients.
• The device should not be connected to both AC and DC power
sources simultaneously.
• In the clinical environment any PC that is used with the flow
generator must be at least 1.5 m away from, or at least 2.5 m
above the patient. It must also comply with IEC 60950 or
equivalent.
Cautions
• At low pressures, the flow through the exhalation ports of your
mask may not clear all exhaled gas from the tubing. Some
rebreathing may occur.
• The air temperature for breathing produced by this device can
be as much as 6ºC higher than the temperature of the room.
Caution should be exercised if the room temperature is warmer
than 32ºC.
•
(S8 series only)
When AC mains power (100–240V AC) is
not available, always use a ResMed DC-12 converter. (The
DC-12 converter is available as an optional accessory. It is not
supplied with all models.)
• Do not remove any attached accessories while power is
connected to your device.
Note:
The above are general warnings and cautions. Specific
warnings, cautions, and notes appear with the relevant
instructions in this user guide.
Servicing
The flow generator should be inspected by an authorised ResMed
service centre five years from the date of manufacture. Before
this, the device is intended to provide safe and reliable operation
if it is operated and maintained according to the instructions
provided by ResMed. Warranty details are provided with the
device at the time of original supply. As with all electrical devices,
if any irregularity becomes apparent, you should have the device
inspected by an authorised ResMed service centre.
Limited Warranty
ResMed warrants that your ResMed flow generator shall be free
from defects in material and workmanship for a period of two
years from the date of purchase by the initial consumer. This
warranty is not transferable.
If the product fails under conditions of normal use, ResMed will
repair or replace, at its option, the defective product or any of its
components. This Limited Warranty does not cover:
a) any damage caused as a result of improper use, abuse,
modification or alteration of the product;
b) repairs carried out by any service organisation that has not
been expressly authorised by ResMed to perform such repairs;
c) any damage or contamination due to cigarette, pipe, cigar or
other smoke;
d) any damage caused by water being spilled on or into the
product.
Warranty is void on product sold, or resold, outside the region of
original purchase. Warranty claims on defective product must be
made by the initial consumer at the point of purchase.
This warranty is in lieu of all other express or implied warranties,
including any implied warranty of merchantability or fitness
for a particular purpose. Some regions or states do not allow
limitations on how long an implied warranty lasts, so the above
limitation may not apply to you.
ResMed shall not be responsible for any incidental or
consequential damages claimed to have occurred as a result
of the sale, installation or use of any ResMed product. Some
regions or states do not allow the exclusion or limitation of
incidental or consequential damages, so the above limitation may
not apply to you. This warranty gives you specific legal rights, and
you may also have other rights which vary from region to region.
For further information on your warranty rights, contact your local
ResMed dealer or ResMed office.
Deutsch
Bitte lesen Sie die auf Ihren Produkttypen zutreffenden
Anweisungen dieser Gebrauchsanweisung. Verwenden Sie die
folgende Liste, um den Produkttypen zu bestimmen:
• APAP – S8™ AutoSet™ Geräte
• CPAP – alle anderen S8 Geräte (außer S8 AutoSet)
• Bilevel – VPAP™ IV und VPAP™ IV ST Geräte.
Indikationen
Ihr Gerät ist für die Verwendung zu Hause und im Krankenhaus
vorgesehen.
CPAP
Ihr CPAP-System ist für die Behandlung von obstruktiver
Schlafapnoe (OSA) bei Erwachsenen vorgesehen.
APAP
Ihr selbstregulierendes Therapiesystem ist für die Behandlung von
obstruktiver Schlafapnoe (OSA) bei Erwachsenen vorgesehen.
Das selbstregulierende Therapiesystem verfügt über zwei
Behandlungsmodi: AutoSet und CPAP mit festgelegtem Druck.
Bilevel
Ihr Bilevel-Beatmungsgerät ist für die nicht-invasive Beatmung
von Patienten mit Ateminsuffizienz bzw. obstruktiver Schlafapnoe
(OSA) vorgesehen.
Kontraindikationen
Positiver Atemwegsdruck kann bei einigen Patienten mit den
folgenden Erkrankungen kontraindiziert sein:
• Pneumothorax oder Pneumomediastinum
• pathologisch niedriger Blutdruck, insbesondere in Verbindung
mit intravaskulärer Volumendepletion
• Liquorausfluss, kürzliche Schädeloperation oder Trauma
• schwere bullöse Lungenerkrankung
• Dehydrierung.
Nebenwirkungen
Verständigen Sie Ihren verschreibenden Arzt bei ungewöhnlichen
Schmerzen in der Brust, starken Kopfschmerzen oder verstärkter
Atemlosigkeit. Bei einer akuten Infektion der oberen Atemwege
kann es sein, dass die Behandlung einstweilig eingestellt werden
muss.
Folgende Nebenwirkungen können während der Behandlung mit
dem Atemtherapiegerät auftreten:
• Trockenheit von Nase, Mund oder Hals
• Völlegefühl
• Ohren- oder Nasennebenhöhlenbeschwerden
• Augenreizungen



